 Zachary Lin – Adapting Galaxy bioinformatics to outbreak-associated Clostridium difficile
Zachary Lin – Adapting Galaxy bioinformatics to outbreak-associated Clostridium difficile
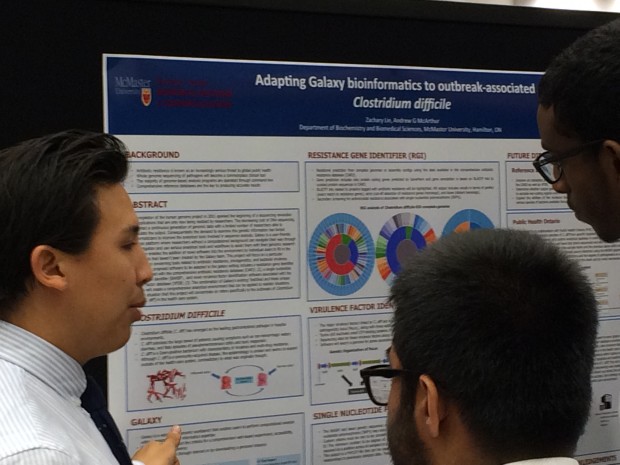
The completion of the human genome project in 2001 sparked the beginning of a sequencing revolution with applications that are only now being realized by researchers. The decreasing cost of DNA sequencing has ignited a continuous generation of genomic data with a limited number of researchers able to manipulate the output. Consequentially the demand to examine this genetic information has forced bioinformaticians to improve the analytical tools involved in sequence analysis. Galaxy is a user-friendly analytical platform where researchers without a computational background can navigate their way through an investigation and use various analytical tools and workflows to assist them with their genomic research (1). Galaxy enables the addition of novel software into the environment by individual users to fill in the gaps of tools that haven’t been created by the Galaxy team. This project will focus on a particular analytical gap concerning tools related to antibiotic resistance, phylogenetics, and bacterial virulence. Currently, the proposed software to be adapted to the galaxy setting includes a resistance gene identifier (RGI) associated with the comprehensive antibiotic resistance database (CARD) (2), a single nucleotide polymorphism identifier (BANSP) , and novel virulence factor identification software associated with the virulence factor database (VFDB) (3). The combination of Galaxy’s existing ToolShed and these unique additions will create a comprehensive analytical environment that can be applied to realistic situations. One such situation that this project will concentrate on refers specifically to the outbreaks of Clostridium difficile (C. diff) in the health care system.
 Kara Tsang – Expansion of the Antibiotic Resistance Ontology for the ESKAPE pathogens
Kara Tsang – Expansion of the Antibiotic Resistance Ontology for the ESKAPE pathogens
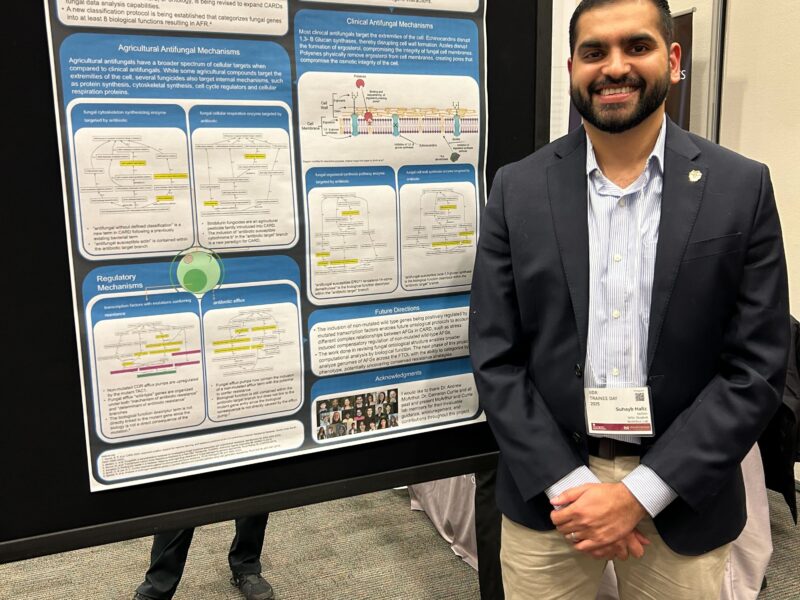
The loss of effective antimicrobials is reducing the ability to protect the global population from infectious diseases, leading to profound impacts on the healthcare system, international trade, agriculture, and environment. The field of antibiotic drug discovery and the monitoring of the dynamic and new antibiotic resistance elements have yet to fully exploit the power of the genome revolution. The curation and directed development of the Comprehensive Antibiotic Database (CARD) will advance the understanding of the genetics, genomics, and threat severity of antibiotic resistance, while simultaneously improving its ability to accurately predict and screen for antibiotic resistance genes within raw genomes. Strategically advancing the Antibiotic Resistance Ontology (ARO), the unique organizing principle of the CARD, allows the value of big data in disparate realms of research to be used and understood by the multidisciplinary efforts working to combat the emergence and prevalence of the ESKAPE pathogens, a critical driving force of the global health crisis.